ATMP
ATMPs
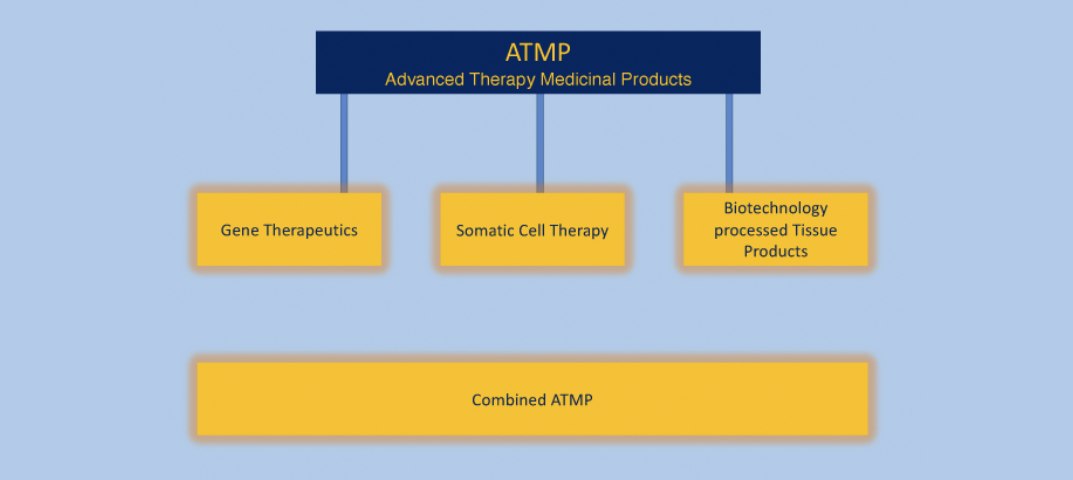
What are ATMPs?
ATMPSs (Advanced Therapy Medicinal Products) are medicines based on living cells or tissues, usually used to treat serious or life-threatening diseases. The use of ATMPs usually requires specialised expertise and facilities to ensure the integrity and efficacy of the products. Examples of ATMPS’s include stem cell therapies, gene therapies and tissue engineering products.
Legal basis for ATMPs in the EU
In the European Union, the European Parliament and the Council adopted Regulation (EC) No 1394/2007 on advanced therapy medicinal products. It has been directly applicable in the Member States since 30 December 2008 (see Art. 30 of Regulation (EC) No. 1394/2007 in conjunction with Art. 288 (2) TFEU - Treaty on the Functioning of the European Union).
Application of ATMPs
The use of ATMPs is usually by transplantation, injection or infusion, the exact method of administration depending on the type of ATMPs. The use of ATMPs usually requires careful monitoring of the patient and close follow-up to identify and treat possible adverse effects in:
Non-reversible joint disease- or trauma.
Certain cancers
Genetic diseases
Severe cardiovascular disease
Requirements
Requirements for ATMPs in medicine and their application are very high, as these products are based on living cells and tissues and are therefore very sensitive.
1. Quality assurance
ATMPS’s must be manufactured according to the highest quality standards to ensure the safety and efficacy of the product. This includes adherence to GMP (Good Manufacturing Practice) guidelines and the implementation of quality control and quality assurance procedures.
2. Safety
ATMPS’s must be safe so as not to endanger patient health. To this end, extensive preclinical studies must be conducted to identify potential adverse effects. Clinical trials then further investigate the safety and efficacy of the product.
3. Effectiveness
ATMPS’s must be effective in helping patients. The effectiveness of the product is tested in clinical trials and must meet the requirements of the relevant regulatory authority.
4. Stability
ATMPS’s must be stable during storage and transport to maintain their effectiveness. Special storage conditions and transport conditions are required for this purpose.
5. Documentation
documentation of the manufacture and use of ATMPs is particularly important to ensure complete traceability and to be able to react quickly in the event of adverse events.
Overall, the requirements for ATMPs are very high in order to ensure the safety and efficacy of these products. The manufacture and use of ATMPs therefore requires specialised facilities and experts to ensure that all requirements are met.