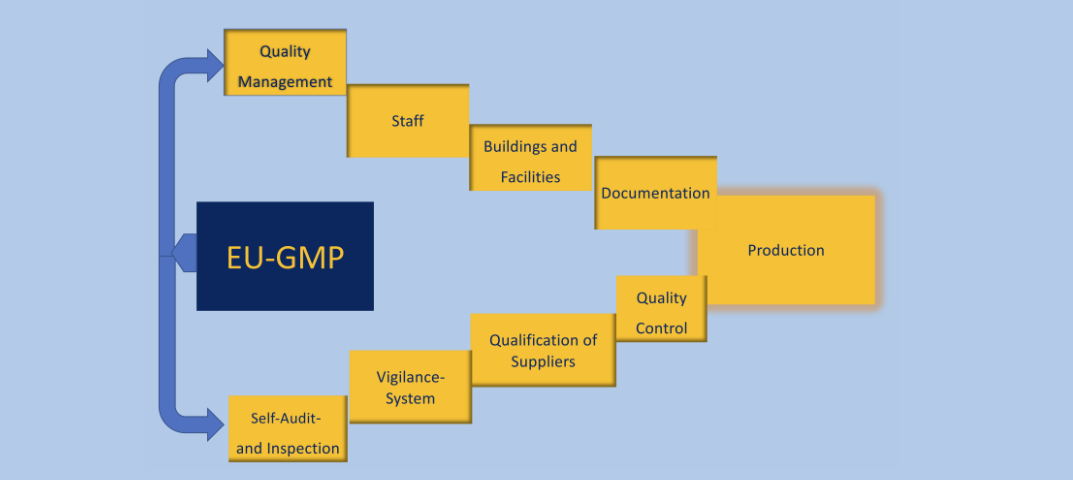
GMP
GMP stands for "Good Manufacturing Practice" and are guidelines for quality assurance in the production of medicines, active ingredients, and medical devices. In the pharmaceutical industry, these requirements are very important to ensure that the products produced are safe, effective and of high quality.
Some of the most important requirements of GMP in the pharmaceutical industry are:
- Documentation
All production processes must be carefully documented, including the manufacturing, packaging, and storage of products.
- Qualification and validation
All production facilities, equipment and processes must be qualified and validated to ensure they meet GMP requirements.
- Training and education
All employees working in the production and control of medicinal products must receive sufficient training and continuing education to perform their duties correctly.
- Cleanliness and hygiene
The production and storage of medicinal products must be carried out under hygienic conditions to avoid contamination and impurities.
- Quality assurance
Appropriate quality assurance measures must be implemented to ensure that products meet specific requirements.
- Traceability
All materials and components used in the production of medicinal products must be traceable.
- Risk management
A risk management process must be implemented to identify and minimise potential risks to the quality and safety of products.
These and other requirements of GMP are essential to ensure the quality and safety of medicinal products and to increase consumer confidence in the pharmaceutical industry.